FDA Authorizes Pfizer-BioNTech Booster for 12-15 Year Olds
On Jan 3, 2022, the FDA revised the current emergency use authorization (EUA) for the Pfizer-BioNTech Covid-19 vaccine. This means that anyone aged 12 and over, who completed the 2 dose series of Pfizer-BioNTech, can now get a booster dose. The timeframe for a Pfizer-BioNTech booster is also updated from 6 months to 5 months.
The FDA amended the vaccine’s EUA for certain immunocompromised people ages 5-11 years. This group of patients (e.g. patients who have undergone solid organ transplantation) is now authorized to have a third primary series dose at least 28 days following the second dose of the two-dose series.
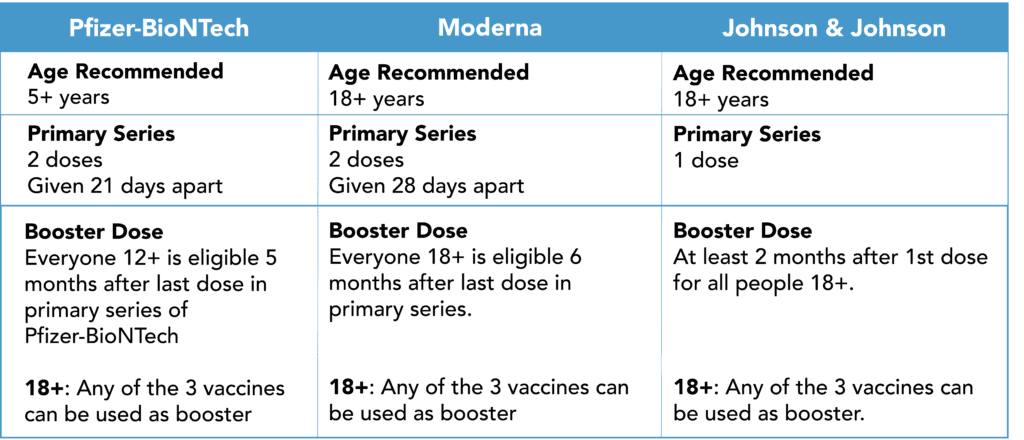
*Click here for additional recommendations for immunocompromised people.
Where can I get a vaccine?
Click here to find where to get vaccinated.